Your pond’s water quality to your fish is as important as our air quality is to us. With that said, do you test your pond water? And if you do regular testing, do you even understand what those tests mean? This article gives you a look into each of the common testing parameters available in most water testing kits.
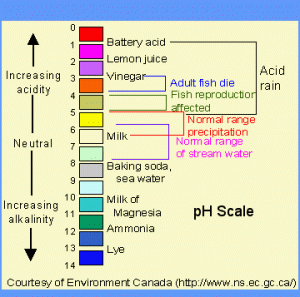
The pH of your water is the amount of hydrogen ions that are present in your water. The pH scale has a range from 0-14, acidic to basic (alkaline), respectively. The midpoint, 7, is considered neutral.
Your pH controls many of the chemical balances in your water. One very important chemical balance is that it affects the toxicity of the ammonia in your pond. In general, the higher your pH and water temperature, the higher the ammonia toxicity will be in your tank (if ammonia is even present).
Ammonia
In your pond, fish will release waste into the water. In addition, decaying plant matter and uneaten fish food will break down. All of these activities will create ammonia. This buildup of ammonia is lethal to fish, especially at escalated pH levels. If left untreated, ammonia will damage delicate gill membranes, causing respiratory distress or death. As the ammonia levels increase, beneficial bacteria named Nitrosomonas will begin to consume and convert the ammonia to nitrite (NO2).
Nitrite
As stated in the previous paragraph, Nitrite (NO2) is the result of beneficial bacteria named Nitrosomonas consuming ammonia and converting it to nitrite. Nitrite is extremely toxic to fish, preventing the fish’s blood from carrying oxygen. This prevents the normal gas exchange from their gills. If severe enough, the fish will die from oxygen starvation. So clearly, this is yet another important parameter at which we should monitor.
If your pond is established and your nitrite level is in check, it’s probably safe to say that you have abundant amounts of the beneficial bacteria named Nitrobacter. It will consume and convert the nitrites to nitrates (NO3). This newly converted nitrate will then be consumed by plants and algae as source of nourishment.
Phosphate
Monitoring your phosphate level is important because it is one of the primary nutrients responsible for algae blooms. Common phosphate sources include decaying plants, waterfowl excrement and treated landscape run-off.
Carbonate Hardness (KH)
Carbonate Hardness, or alkalinity, is the measurement of the water’s ability to neutralize acid, known as the buffering capacity. Buffering capacity is the water’s ability to keep the pH stable as acids or bases are added. So if the water has sufficient buffering capacity, it can absorb (like a sponge) and neutralize additions without affecting the pH. There is, however, a limit on the sponge’s ability. Once this limit is reached, the pH can change rapidly.
Carbonate Hardness is also an important source of energy for nitrifying bacteria that consume ammonia and nitrite.
General Hardness (GH)
General Hardness, or Total Hardness (TH) is a measurement of the dissolved salts in the water, mostly composed from calcium and magnesium. The concentration of dissolved salts is important to fish for 2 reasons: Osmotic regulation (equilibrium of the internal salt concentration) and blood calcium levels are regulated by the amount of General Hardness levels.